Organic revenue growth of 13% in Q2 builds on strong Q1 performance Good momentum and strong order intake provide good visibility for second half Firmly on track to deliver double digit revenue growth with high single-digit growth in adj. EBITDA […]
Press releases worldwide
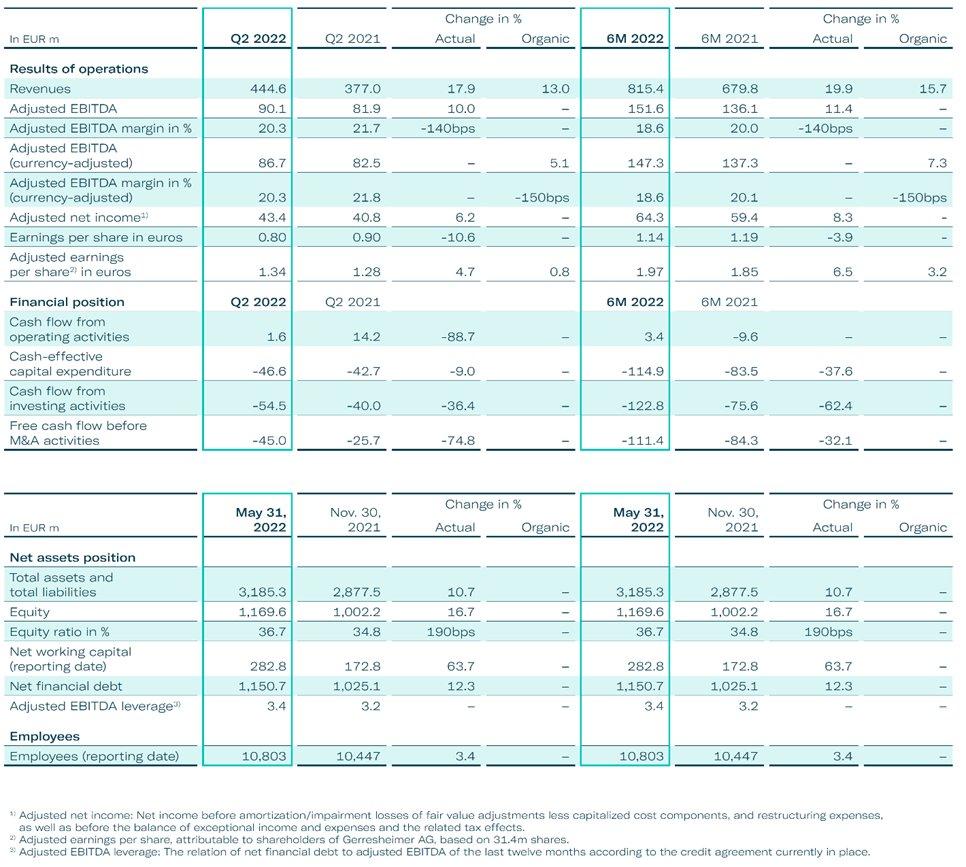
Organic revenue growth of 13% in Q2 builds on strong Q1 performance Good momentum and strong order intake provide good visibility for second half Firmly on track to deliver double digit revenue growth with high single-digit growth in adj. EBITDA […]
Gerresheimer has been awarded the gold medal for the first time by EcoVadis, one of the leading providers of sustainability rankings, for the successful implementation of its sustainability strategy. With 68 out of a maximum of 100 possible points, Gerresheimer […]

Gerresheimer has assumed responsibility for the industrialization of a dry powder inhaler for the treatment of respiratory ailments for MERXIN (United Kingdom), a company that specializes in making inhaler devices. The inhaler is produced in Pfreimd (Germany) for worldwide distribution. […]
Partners join forces to improve lives of millions of Parkinson’s patients worldwide with a transformative technology-based solution Gerresheimer participates in seed-round of the Finnish MedTech company and university spin-off Monitoring and personalized medication adjustment in combination is a novum Gerresheimer […]
The Annual General Meeting of Gerresheimer AG has approved the payment of a dividend of EUR 1.25 per share for the 2021 financial year. This represents a payout ratio of 30 percent. “2021 was a record year for us. Our […]
The drug development company ISR has signed an agreement with Gerresheimer for large-scale production of the IcoOne nasal inhaler, for its phase III study of its dry powder Covid-19 vaccine. Gerresheimer is an established company with global capacity for the […]
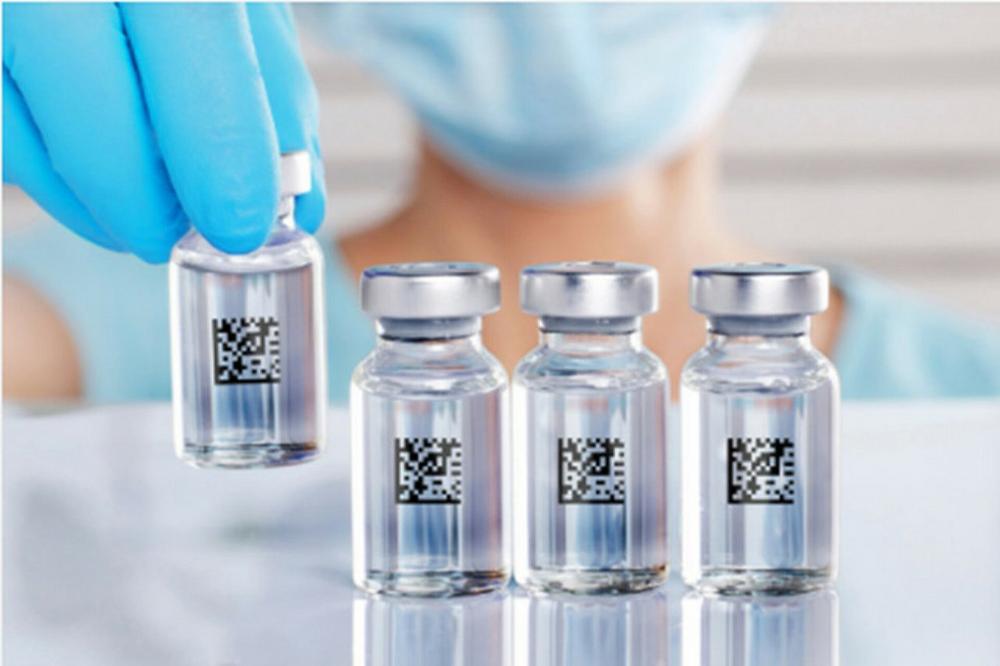
The Gerresheimer traceability concept ensures complete transparency throughout the value chain and greater safety for the patient — because only complete traceability is true traceability. As the first link in the manufacturing chain, the company applies unique codes to its […]
. – Partners are developing needle-free drug delivery platform for biologics and biosimilars – This Innovative solution improves patient’s quality of life, especially for people with chronic diseases – Partners will together expand their client base, product range and IP […]
First-quarter revenue grew organically by 19.1% Adjusted EBITDA up organically by 10.6% Gerresheimer raises revenue guidance to double-digit growth for 2022 Gerresheimer AG, a leading provider of healthcare & beauty solutions and drug delivery systems for pharma, biotech and cosmetics, […]
Pharma and medical technology specialist Gerresheimer has been awarded platinum status by its customer Chiesi. Classification as an especially high-quality supplier is based on a rating process carefully examining sustainability, economy, quality, innovation and service level. Gerresheimer secured a top […]